The Allergy, Genes and
Environment
Network (AllerGen)
OUTCOMES
& IMPACTS
2004 - 2021


– Dr. Kelly McNagny, The University of British Columbia
AllerGen Associate Scientific Director, GxE leaderMessage
from the Board Chair, Scientific Director and President & CEO
After receiving the full 14 years of federal Networks of Centres of Excellence (NCE) funding, and an additional two years of support to mobilize knowledge and commercialize research results, the Allergy, Genes and Environment (AllerGen) Network completed its NCE term on March 31, 2021.
In this report, we take the opportunity to look back on our Network’s contributions and achievements since 2004.
Credit for these incredible accomplishments rests with AllerGen’s researchers, trainees and partners from across the country and around the world. They have worked together in a collaborative, coordinated, and networked effort to benefit those living with and caring for individuals with allergy, asthma and related immune disease. Their passion and commitment have generated new knowledge, advanced drug development, forged strong national and global communities in allergy and asthma research and innovation, and provided clinical and professional development training opportunities that have nurtured future generations of leaders in related fields.
AllerGen extends its deep appreciation to the Networks of Centres of Excellence (NCE) Program; AllerGen’s Board of Directors; Research Management Committee (RMC); Scientific, Training and Commercialization/KTEE advisory committees; the Administrative Centre team; and AllerGen’s host institution, McMaster University, for invaluable guidance, support and championing of AllerGen’s vision, mission and goals.
Together, we have transformed the landscape of allergic and respiratory disease in Canada and leave a lasting and self-sustaining legacy of national and global impacts that will perpetuate and increase the momentum achieved through the government of Canada’s NCE program for generations to come.
Thank you to all and may the “network way of working” continue to guide your future endeavours!

Pieter Cullis
Board Chair
Judah Denburg
Scientific Director
Diana Royce
President & CEO
– Dr. Meghan Azad, The University of Manitoba
Deputy Director, CHILD Cohort Study
Executive summary
The Allergy, Genes and Environment Network (AllerGen) was established in 2004 to unite Canada’s allergic and respiratory disease communities with the overall goal of improving the lives of Canadians living with asthma, allergies, anaphylaxis and related immune diseases.
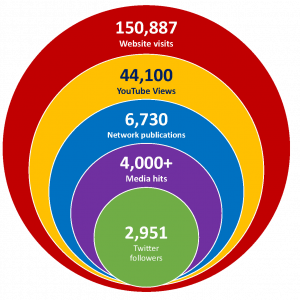
Over 15 years as a national Networks of Centres of Excellence, AllerGen invested over $51M in 220 research projects; trained 1,763 Highly Qualified Personnel (HQP) students and trainees; partnered with 651 organizations across sectors to leverage $128.6M; generated 6,730 scientific publications; and has had its research featured in major national and international media over 4,000 times.
Through the formation of partnered, trans-disciplinary, multi-sectoral teams, sharing a drive for unmitigated research excellence, AllerGen has created valuable new knowledge, products and tools; enabled knowledge and technology uptake and application by receptor and stakeholder communities in Canada and beyond; and built much needed national clinical capacity in allergic /asthmatic disease prevention and management.
Through its strategic research investments, AllerGen catalyzed three ongoing, self-sustaining Legacy Initiatives that benefit those living with—and caring for people affected by—allergy, asthma and related immune diseases.
Executive Summary
CHILD Cohort Study (CHILD)
In 2008, AllerGen launched CHILD as a national discovery platform. This pan-Canadian, general population-based longitudinal birth cohort study is one of only a few global initiatives that enable investigation of the early-life origins of asthma, allergy, and other chronic non-communicable diseases (NCDs).
The CHILD team has generated over 100 scientific publications and delivered break-through findings on the impact of early-life exposures (e.g., mode of delivery, breast-feeding, antibiotics, air pollution, pets, household cleaning products, sleep, screen-time, food and nutrition) on the development of childhood allergies, asthma, obesity, cognitive capacity, the immune system and the internal microbiome.
Impactful CHILD results have been reported over 2,000 times by global media outlets (TIME Magazine, People, CNN, New York Times, CBC, Globe & Mail, Maclean’s, and many others), informing policy, practice and the advocacy efforts of organizations such as UNICEF Canada and The Sandbox Project, healthcare professionals, and dozens of grassroots maternal/child health organizations and stakeholders.
CHILD has produced data critical to hypothesis-generation and novel basic and applied research initiatives in the field of maternal-child health.
CHILD data will also fuel novel research opportunities for decades to come.

Mikaela, 8 years (CHILD participant in Vancouver, BC): “My poster is about all the good things the CHILD Cohort Study has done.”
Clinical Investigator Collaborative (CIC)
To accelerate drug development for the treatment of asthma and other inflammatory airways diseases, AllerGen established the CIC, a multisite Phase II clinical trials consortium operating as an academic clinical research organization (A-CRO) at six Canadian universities and one international site—the Karolinska Institute in Stockholm, Sweden.
Led by world-renowned scientists, the CIC has conducted 29 clinical trials with 19 industry partners, including global pharmaceutical enterprises AstraZeneca, Genentech, Novartis and Pfizer, and new Canadian biotechnology companies Asmacure and AIM Therapeutics.
CIC trials have attracted nearly $30 million in R&D investment to Canada and created over 41 jobs for scientists, trainees and research associates, positioning Canada as a global leader in the development of allergic asthma therapeutics.
For example, the CIC identified the new biologicals Ligelizumab (anti-IgE, Novartis), Quilizumab (anti-IgE, Genentech Inc.) and Mepolizumab (anti-IL-5, GlaxoSmithKline), as molecules for future investment to treat various forms of asthma.
In 2014, the CIC studied the biologic drug Tezepelumab (anti-TSLP, Amgen) and first identified the drug’s significant therapeutic potential to treat allergic asthma. The findings were published in The New England Journal of Medicine, reported broadly in major media outlets, and set the stage for rapid clinical development of the molecule hailed as a “blockbuster drug.”
National Food Allergy Strategic Team (NFAST)
To improve the quality of life of Canadians affected by food allergies, AllerGen established a unique translational research program focused on understanding the causes, prevalence, treatment and consequences of food allergy and anaphylaxis. NFAST—comprising clinical, natural and social scientists working in trans-disciplinary, inter-institutional teams –has enabled better clinical management strategies, created innovative educational tools, and advanced policy and public health measures addressing food allergy.
With 138 refereed publications since 2007, NFAST teams and their partners across sectors have:
- produced Canada’s first food allergy prevalence data, informing Health Canada’s food allergen labelling reform initiative in 2012 and contributing to provincial legislation that protects students at risk of anaphylaxis;
- created the first national anaphylaxis surveillance database that provides critical information on what causes anaphylaxis, how often it occurs, whom it affects, and how it is being treated across provinces;
- supported emerging clinical therapies such as food allergy oral immunotherapy (OIT) and produced new findings on the genetic basis of food allergy; and
- created innovative, award-winning educational tools, food allergy apps and peer-to-peer mentoring programs for kids with food allergies.
Highly Qualified Personnel (HQP) Program
AllerGen’s Highly Qualified Personnel (HQP) program has been one of the Network’s most impactful and transformational achievements.
From 2005 to 2019, AllerGen invested $26.4 million in HQP programming ($22.3 million in research support and $4.1 million in specialized awards, grants and Fellowships) to provide advanced education, training and capacity-building opportunities to 1,763 trainees, research staff and early-career professionals.
Of the Network’s HQP graduates, at least 328 are now employed across sectors including industry, policy development, healthcare and academe; 14 advanced to become Network investigators; and 74 (4.2%) secured faculty positions in Canada and abroad.
In addition to value-added HQP awards, studentships and training opportunities, AllerGen established a prestigious, two-year Emerging Clinician Scientist (ECS) Fellowship to support the development of the next generation of Canadian clinical immunologists and allergists in their pursuit of research training and a combined career as clinicians and academic researchers.
Through this program, AllerGen invested $1.25 million in support of five outstanding clinician-scientists, who are now ideally positioned to translate and transform research knowledge into improved patient care and health for the benefit of Canadians.
Enabling Platforms
AllerGen’s Legacy Initiatives have been complemented by three Enabling Platforms that have produced investigative breakthroughs in biomarkers, bioinformatics, gene-environment interactions and personalized health, and enabled improved policies and clinical care/management practices in asthma and allergies.
The Platforms have provided a strategic connection to research technologies and methods, ensuring that AllerGen discoveries continue to be both novel and relevant to industry, clinicians and policymakers.
Partnerships
Network partners have played a vital role in shaping AllerGen’s research outcomes, and in translating and commercializing findings that bridge the gap between the lab and Canadians living with allergic and asthmatic disease.
Since 2005, Network-wide partner cash and in-kind investments total more than $128.6M, representing an NCE to non-NCE leveraging of $1 : $1.75.
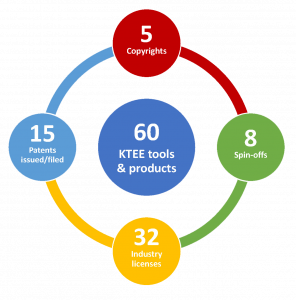
Knowledge & Technology Exchange & Exploitation
AllerGen’s knowledge mobilization and commercialization activities have accelerated the uptake and application of Network research outputs by partners, stakeholders and receptor communities in Canada and abroad.
Poised to address new health priorities
AllerGen’s 15-year commitment to networked, collaborative research across disciplines has positioned its pan-Canadian teams to contribute to global efforts to address new and evolving health priorities across the entire span of immune/ inflammatory disorders and responses.
Currently, AllerGen investigators are leveraging the network structure to mount rapid responses to address the COVID-19 pandemic and CHILD is conducting an important add-on study to measure the direct impacts of COVID-19 infection, as well as the indirect emotional and socioeconomic impacts of public health containment strategies on Canadian families.
AllerGen NCE wishes to thank the Government of Canada for the opportunity to build this Networks of Centres of Excellence over the past 15 years and is proud t0 confirm that AllerGen has had a significant and lasting impact on the Canadian landscape of discovery, innovation and translation that will endure for generations to come.
As of April 1, 2020, AllerGen’s Administrative Centre continues to operate as a not-for-profit organization supporting two of the Network’s three Legacy Initiatives – CHILD and the CIC. AllerGen partner organizations the Canadian Society of Allergy and Clinical Immunology (CSACI) and Food Allergy Canada are providing leadership and continuity to AllerGen’s third Legacy Initiative, founded on the Network’s food allergy research investments, towards establishing a national food allergy strategy for Canada.
Research Projects
(n=220)
No Data Found
Researchers
(n=528)

No Data Found
HQP
(n=1,763)

No Data Found
Partners
(n=651)

No Data Found

“The support of AllerGen NCE had been crucial to my allergy program success. At the onset of my career, I was awarded the first AllerGen Emerging Clinician-Scientist Fellowship award which allowed me to establish a strong foundation for my subsequent research program. With AllerGen’s continuous support for the C-CARE anaphylaxis and food allergy registry, I have addressed new research questions that bridge knowledge gaps and contribute to the improved management of patients with severe allergies.“
– Dr. Moshe Ben-Shoshan, McGill University
Corporate profile
AllerGen was established in 2004 by Innovation, Science and Economic Development Canada (formerly Industry Canada) through the Networks of Centres of Excellence (NCE) Program to reduce the burden of allergic disease through leadership in research, knowledge translation and exchange, and trainee capacity building.
AllerGen has formed an enduring national research network of scientists, clinicians, stakeholders and partners in industry, government and healthcare working together to help Canadians tackle the challenges of living with asthma, allergies, anaphylaxis and related immune diseases.
NETWORK PERFORMANCE (2005-2021)
“AT A GLANCE”
AllerGen’s VISION
To create an enduring network of allergy and immune disease experts whose discovery and development efforts contribute to reducing the impact of allergic and related immune diseases nationally and globally.
AllerGen’s MISSION

– Dr. Jennifer Protudjer, University of Manitoba
integrated research strategy
An evolution to lasting legacies
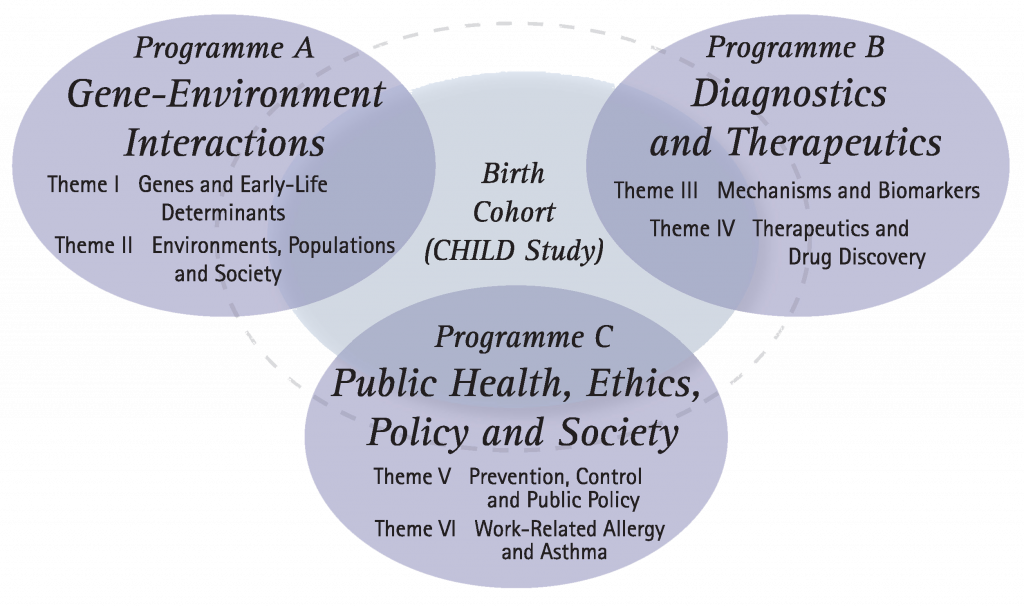
Establishing an allergic disease research framework (2005)
AllerGen’s initial research program was structured around six themes within three programmatic thrusts, representing key elements of the Network’s expertise in the field of asthma and allergies.
Internationally recognized research leaders coordinated the identification and realization of strategically important research opportunities within each theme. AllerGen supported 34 inaugural projects from which a national birth cohort, the Canadian Healthy Infant Longitudinal Development (CHILD) Study, emerged.
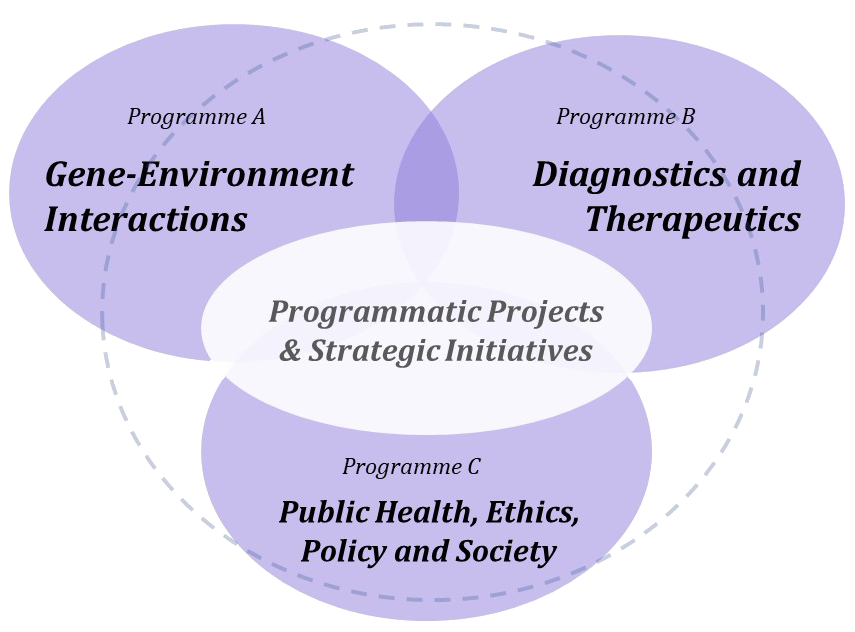
Enhanced integration and collaboration (2007)
Three years into its NCE mandate, AllerGen consolidated its six research themes into three cross-disciplinary research programs, enhancing focus and collaboration across the Network.
This model supported 39 active research projects and provided seed funding for strategic initiatives to catalyze new investigative teams and projects aligned with the Network’s Knowledge and Technology Exchange and Exploitation (KTEE) priorities.
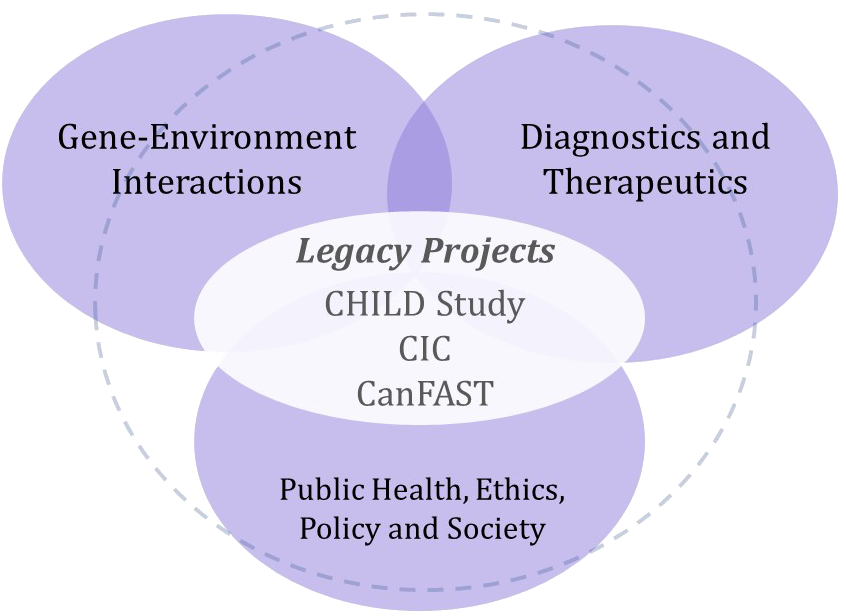
Building research legacies (2012)
In 2012, AllerGen completed the first of two seven-year NCE funding cycles, investing nearly $25 million in 127 research projects and strategic initiatives since 2005.
The research program further evolved into a fully integrated research strategy built upon three Legacy Projects supported by three Enabling Platforms.
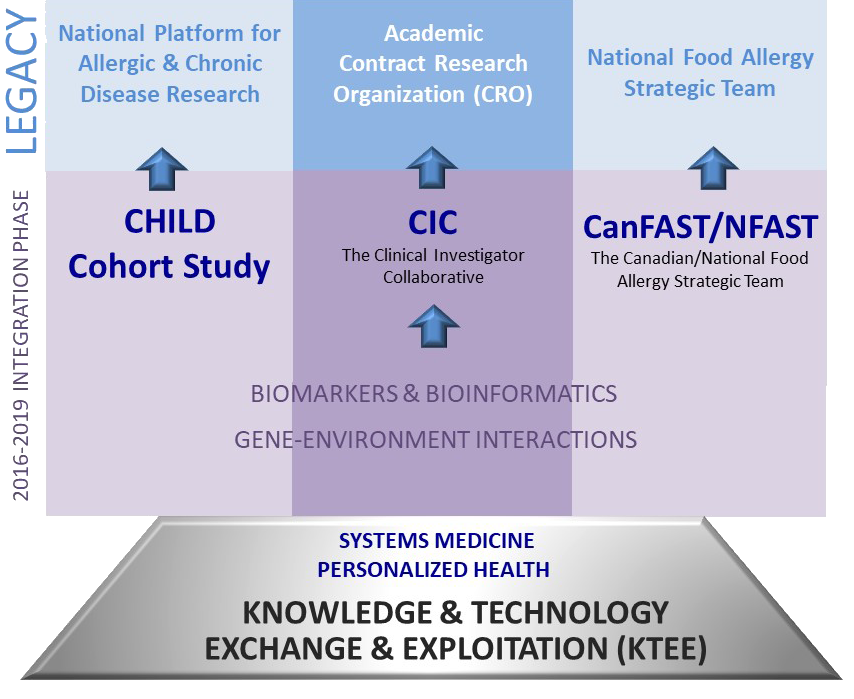
Lasting legacies for Canada (2016-2019)
By 2019, AllerGen’s research strategy culminated in three self-sustaining Legacy Initiatives that represent a lasting legacy for Canada’s investment in this national Networks of Centres of Excellence in allergic disease.
AllerGen’s Enabling Platforms were integrated into these Legacy Initiatives and/or wrapped up their research projects.

– Laura Feldman, University of Toronto
financial overview
From 2005-2020, AllerGen’s total NCE budget was $73.53M. Since 2005, Network-wide partner cash and in-kind investments total more than $128.6M, representing an NCE to non-NCE leveraging of $1 : $1.75.
Leveraging ratio 2005-2020
(NCE$ : Non-NCE$)

No Data Found
In the second half of AllerGen’s NCE mandate (2012-2020), when the Network’s final research strategy was established, the distribution of research investments by platform became proportionate to their anticipated legacy impacts.
Research investment by platform
(2012-2020)

No Data Found
Establishing an allergic disease research framework (2005)
Gene-Environment Interactions
Theme I:
Using data from the Saguenay-Lac St. Jean Quebec Founder Cohort and international birth cohort studies, AllerGen investigators identified genes associated with susceptibility to allergic and immune diseases, and developed, validated and implemented a microarray genotyping diagnostic tool called “AllerChip”.
Theme II:
AllerGen researchers studied the genes involved in allergic disease within the context of gene-environment interactions, enabling the development of individual-specific susceptibility factors, prevention strategies and the identification of personalized therapeutic targets.
Dr. Peter Paré
University of British Columbia
Research leader

Dr. Malcolm Sears
McMaster University
Research leader
Diagnostics and Therapeutics
Theme III:
Investigators explored the role of pathogen-host interactions in allergy/asthma (A/A); the molecular pathology of A/A; the initiation, induction and expression of the allergic cascade; and novel neuroimmune (mind-body) interactions in A/A—enabling new and improved diagnostic tools and tests, new therapeutics and more effective use of existing therapeutics.
Theme IV:
AllerGen launched the Clinical Investigator Collaborative (CIC)—a national clinical trials consortium consisting of four Canadian research centres.
The CIC evaluated the potential effectiveness of new molecules proposed to treat airway inflammation, and simultaneously studied the mechanics of allergic disease.

Dr. Dean Befus
University of Alberta
Research leader

Dr. Paul O’Byrne
McMaster University
Research leader
Public Health, Policy and Society
Theme V:
AllerGen supported a range of research designed to have positive impacts on public health, policy and society.
One project produced a Financial Barrier Risk Index to illustrate the effects of financial barriers on asthmatic children; another showed that the lack of a drug plan and low socioeconomic status directly affect asthma health outcomes.
Theme V projects provided new insights to health policymakers that improved child health and enabled healthcare system decision-makers to better target services.
Theme VI:
Investigators focused on projects designed to improve work-related allergy and asthma, including a surveillance of occupational asthma; a study of asthma and BC workers; and an investigation of workplace sensitizers, responses and prevention.

Dr. Allan Becker
University of Manitoba
Research leader

Dr. Susan Elliott
McMaster University
Research leader
Enhanced integration and collaboration (2007)
Program A: Gene-Environment Interactions
AllerGen’s Gene-Environment Interactions teams:
- studied the effects of the environment on an individual’s genetic makeup and the associated development of allergic disease and asthma;
- developed an allergen-gene environment database resource;
- conducted a pan-Canadian study of gene-environment interactions in the etiology of occupational asthma; and
- designed three-dimensional online animations to teach academic, clinical and lay audiences about genetics.

Dr. Jeffrey Brook
Environment Canada, University of Toronto
Research leader
Dr. Peter Paré
University of British Columbia
Research leader
Program B: Diagnostics and Therapeutics
Researchers further advanced the development of asthma and allergy biomarkers, examining different traits of genes, blood and urine as possible indicators of disease.
Program B also established a comprehensive portfolio of food allergy research, from the genetics of food allergy through to clinical management. The Canadian Group on Food Allergy Research (CanGoFAR) investigators focused on understanding fundamental issues that determine how food allergies develop and on finding new ways to prevent sensitization to foods and induce food tolerance.
The CIC continued to expand Canada’s global leadership in allergic asthma drug testing and discovery. By 2007-08, the CIC was networked across five Canadian sites and had completed seven clinical trials with pharmaceutical and biotechnology clients, representing a value of over $6.3 million to Canadian research and development.

Dr. Dean Befus
University of Alberta
Research leader

Dr. Paul O’Byrne
McMaster University
Research leader
Program C: Public Health, Policy and Society
Investigators in Public Health, Ethics, Policy and Society developed a range of allergic disease management and surveillance tools, including:
- a school-based asthma education program for children;
- an e-learning certificate course on food allergy and anaphylaxis; and/li>
- the Respiratory Global Research and Training (GReAT) Network – an international repository for chronic respiratory disease prevalence data to help national and international health decision-makers monitor and improve respiratory health.

Dr. Allan Becker
University of Manitoba
Research leader

Dr. Susan Elliott
McMaster University
Research leader
CHILD Study
In 2008, in partnership with CIHR, AllerGen launched the Canadian Healthy Infant Longitudinal Development (CHILD) Study as a national discovery platform. Led by Dr. Malcolm Sears (Director) and Dr. Padmaja (PJ) Subbarao (Deputy Director), a team of Principal Investigators from across multiple disciplines and spanning the Network’s three programs of research began to investigate the genetic and environmental factors influencing the development of asthma and allergies in children.
CHILD is the largest multidisciplinary, longitudinal, general population-based birth cohort study in Canada, following the lives of nearly 3,500 children, and their parents, over time as they grow – from mid-pregnancy into childhood, adolescence and beyond.
From its inception, CHILD became an overarching and unifying focus for the full spectrum of Network-supported research.

Dr. Malcolm Sears
McMaster University
Director

Dr. PJ Subbarao
SickKids Hospital
Deputy Director
Building research legacies (2012)
Legacy Project:
The Canadian Healthy Infant Longitudinal Development (CHILD) Study
In 2008, CHILD began collecting immunological, environmental, physiological and genetic data from nearly 3,500 Canadian children from pre-birth to school age and beyond, to inform research about the early-life factors influencing the development of asthma, allergies and other chronic immune and inflammatory diseases.
In 2012, peer-reviewed publications of CHILD’s findings began to emerge.
In early 2013, gut microbiome research using CHILD Study samples garnered international attention and highlighted the potential impact of early childhood exposures, such as the method of delivery in childbirth and the method of infant feeding, on lifelong health.

Dr. Malcolm Sears
McMaster University
Director

Dr. PJ Subbarao
SickKids Hospital
Co-Director
Legacy Project:
The Clinical Investigator Collaborative (CIC)
Between 2005 and 2012, AllerGen’s Allergic Asthma-CIC undertook 18 clinical trials with industry partners valued at nearly $17 million. With an AllerGen investment of $2.9 million during this period, the CIC achieved an NCE to non-NCE leveraging ratio of $1:$5.82.
In 2012, the CIC expanded its clinical trials to include studies focused on severe asthma (SA-CIC) and allergic rhinitis (AR-CIC).

Dr. Paul O’Byrne
McMaster University
CIC – Allergic Asthma

Dr. Param Nair
McMaster University
CIC – Severe Asthma

Dr. Anne Ellis
Queen’s University
CIC – Allergic Rhinitis
Legacy Project:
The Canadian Food Allergy Strategic Team (CanFAST)
The Canadian Group on Food Allergy Research (CanGoFAR) teams and projects, from previous years’ Program B: Diagnostics and Therapeutics, matured into the Canadian Food Allergy Strategic Team (CanFAST) – an innovative, nationally networked approach to food allergy and anaphylaxis research.
CanFAST findings came to inform our understanding of the origins, causes, prevalence and treatment of food allergy and enabled the development of improved clinical management strategies and new public health measures.

Dr. Ann Clarke
McGill University
Research leader

Dr. Jean Marshall
Dalhousie University
Research leader
Enabling Platform:
Gene-Environment Interactions (GxE)
The GxE Enabling Platform continued its focus on genetic, environmental and epigenetic research with the goals of discovering novel therapies and diagnostics, and implementing novel public health interventions and policies related to managing the risks associated with asthma and allergies.
As a step towards creating a low-cost, portable system for real-time monitoring of air pollution, GxE investigators created and tested prototype devices equipped with an array of sensors to monitor local concentrations of nitrogen oxide (NO2), ozone (O3) and particulate matter (PM2.5) to help persons living with allergic disease to better identify and manage their environmental exposures.

Dr. Andrew Sandford
The University of British Columbia
Research leader

Dr. Jeffrey Brook
Environment Canada, University of Toronto
Research leader
Enabling Platform:
Biomarkers and Bioinformatics (B&B)
B&B researchers used standard operating protocols and animal models to develop an integrated, world-leading systems biology approach to biomarker development and commercialization.
One B&B team developed a blood test that accurately predicts late-phase allergic responses, while another developed a novel technique to study a rare biomarker.

Dr. Kelley McNagny
The University of British Columbia
Research leader

Dr. Dean Befus
University of Alberta
Research leader

Dr. John Gordon
University of Saskatchewan
Research leader
Enabling Platform:
Patients, Policy and Public Health (PPP)
With an increasing focus on patients and knowledge users, the PPP Enabling Platform leveraged the Network’s research expertise to generate new knowledge, products and services that inform public policy, public health practices, patient and health professional outreach, and educational disease management tools.

Dr. Allan Becker
University of Manitoba
Research leader
Discovery Legacy
CHILD Cohort Study (CHILD)
Formerly: Canadian Healthy Infant Longitudinal Development (CHILD) Study
Key impacts
From discovering how an infant’s genetics, environment, and early-life exposures influence the development of allergies, asthma and obesity, to gaining better insight into how breastmilk and antibiotics shape the infant gut microbiome, to linking delayed food introduction with an increased risk of food sensitization, the innovative work of CHILD researchers is helping to predict, prevent and treat chronic disease.
CHILD has published over 100 articles in peer-reviewed academic journals and become a trusted source of child health knowledge for a wide range of stakeholders, providing parents, clinicians, not-for-profit organizations, policymakers and communities.
CHILD has also collected over 40 million data points, 600,000 questionnaire responses and 500,000 biological samples. Its vast datasets are housed in CHILDdb, a unique, interactive platform under development that will soon be available to researchers worldwide, enabling future discoveries.

Dr. PJ Subbarao
SickKids Hospital
Director

Dr. Stuart Turvey
The University of British Columbia
Co-Director
The next five years …
In 2018, CHILD achieved a major milestone with the completion of its original endpoint: all 5-year-old participants’ clinical visits and a participant retention rate of 93.3%. CHILD has since transformed into a longer-term study, with plans to conduct clinical assessments at ages eight, 12 and 15 to identify critical disease intervention “windows” from preconception to adolescence.
By 2025, CHILD anticipates fueling significant new child health advances, including:
- discovery of precise biomarkers to predict persistent asthma, with translation into novel therapeutics;
- tracking lung function growth from infancy to adolescence and discovery of factors promoting lung health;
- understanding the gender switch in asthma to female predominance during puberty;
- identifying the role of the early-life environment in the development of obesity and cardiovascular /metabolic syndrome risk and disease factors; and
- informing the new field of epigenetics.
Commercialization Accelerator Legacy
Clinical Investigator Collaborative (CIC)
Key impacts
The CIC has attracted nearly $30 million in R&D investment to Canada and created over 41 jobs for scientists, trainees and research associates. Led by renowned experts at McMaster University, the CIC operates at six Canadian universities and one international site.
Since 2005, the CIC has conducted 29 “proof-of-concept” clinical trials that determine pharmacological activity, pharmacokinetics, target engagement, and efficacy of new molecules and compounds for airways diseases, and has licensed 23 Standardized Operating Practices (SOPs) to industry partners.

Dr. Paul O’Byrne
McMaster University
CIC – Allergic Asthma

Dr. Gail Gauvreau
McMaster University
CIC – Allergic Asthma

Dr. Param Nair
McMaster University
CIC – Severe Asthma
The next five years …
Beyond 2020, the CIC will continue testing new compounds, maintaining Canada’s position as a leader in the discovery, development and commercialization of new tests and treatments for the benefit of individuals suffering from airway diseases.
Patients, policy & public health legacy
National Food Allergy Strategic Team (NFAST)
Key impacts
AllerGen’s transdisciplinary NFAST research teams have made significant contributions to understanding the causes, prevalence, treatment and consequences of food allergy and anaphylaxis. This team has also informed important new national regulatory changes with respect to food labelling and the development of food industry guidelines for allergen thresholds; produced Canada’s first food allergy prevalence data; established the first Canadian anaphylaxis surveillance database; created innovative educational tools; and supported emerging clinical therapies.
Finally, NFAST has laid the foundation for the development and implementation of a coordinated, comprehensive national food allergy strategy to be carried forward by AllerGen’s clinical and community partner organizations, the Canadian Society of Allergy and Clinical Immunology (CSACI) and Food Allergy Canada.

Dr. Ann Clarke
McGill University
Research leader

Dr. Susan Elliott
University of Waterloo
Research leader

Dr. Jean Marshall
Dalhousie University
Research leader
The next five years …
NFAST researchers are poised to publish new data on the economic impact of food allergy in Canada; issue guidance to industry to assist in managing allergen precautionary labelling (i.e. food products with a “may contain” statement); and update food allergen exposure data along with recommendations for practical allergen sentinel levels. They will also release findings on the factors that contribute to a safer environment for students at post-secondary institutions in Canada and produce a genetic/epigenetic risk index for peanut and other food allergies.
Enabling Platforms
KEY IMPACTS
In 2017, AllerGen’s Patients, Policy and Public Health (PPP) Enabling Platform became fully integrated into the Network’s CHILD Cohort Study and National Food Allergy Strategic Teams (NFASt) Legacy Initiatives.
Together, AllerGen’s Enabling Platforms provided further evidence to inform the Developmental Origins of Health and Disease (DOHaD) hypothesis that the environment before birth and in the early years of life, including nutrition, exposure to toxic stress and environmental chemicals, impacts lifelong risk for chronic diseases like obesity, hypertension and diabetes.
Gene-Environment Interactions (GxE)
Key Impacts
The GxE Enabling Platform generated important discoveries about the contribution of genes, gene-environment interactions and environmental exposures to allergic diseases and asthma.
GxE findings also aided the development of novel therapies, diagnostics and public health interventions for these conditions.

Dr. Jeffrey Brook
Environment Canada, University of Toronto
Research leader

Dr. Michael Kobor
The University of British Columbia
Research leader
Biomarkers and Bioinformatics (B&B)
Key Impacts
B&B Enabling Platform research helped advance the era of “personalized health” by identifying and developing biomarkers to predict and monitor allergy/asthma, and by leveraging bioinformatics technology to link vast datasets from across the Network, facilitating global studies of allergic disease.

Dr. Kelley McNagny
The University of British Columbia
Research leader

Dr. Dean Befus
University of Alberta
Research leader

Dr. John Gordon
University of Saskatchewan
Research leader

